About AONs
Antisense oligonucleotides (AONs) are synthetic molecules that can bind to pre-mRNA and as a consequence interfere with pre-mRNA splicing. Therapeutically, AONs can be employed in various ways to correct splicing defects resulting from genetic mutations, to induce the in frame skipping mutated native exons, or modulate gene expression via RNA silencing.


Our scientific approach for treatment of Stargardt disease and Usher syndrome
The versatility of AONs allows these molecules to be used in a number of different ways. The most straightforward use of AONs – which is also applied in Astherna’s first AON therapy for Stargardt disease – is to prevent the recognition of pseudoexons. Radboudumc scientists have therefore developed AONs for the treatment of Stargardt disease, by correcting various mutant ABCA4 transcripts. An alternative approach – which is applied for Astherna’s second AON therapy for Usher syndrome – is to induce the in frame skipping of native mutated exons. Radboudumc have developed AONs that induce the skipping of specific USH2A target exons. Astherna builds on the inventions and knowledge gathered during years of research by the Radboudumc, to now bring novel therapeutics to patients.
How do Astherna’s AON therapies work?
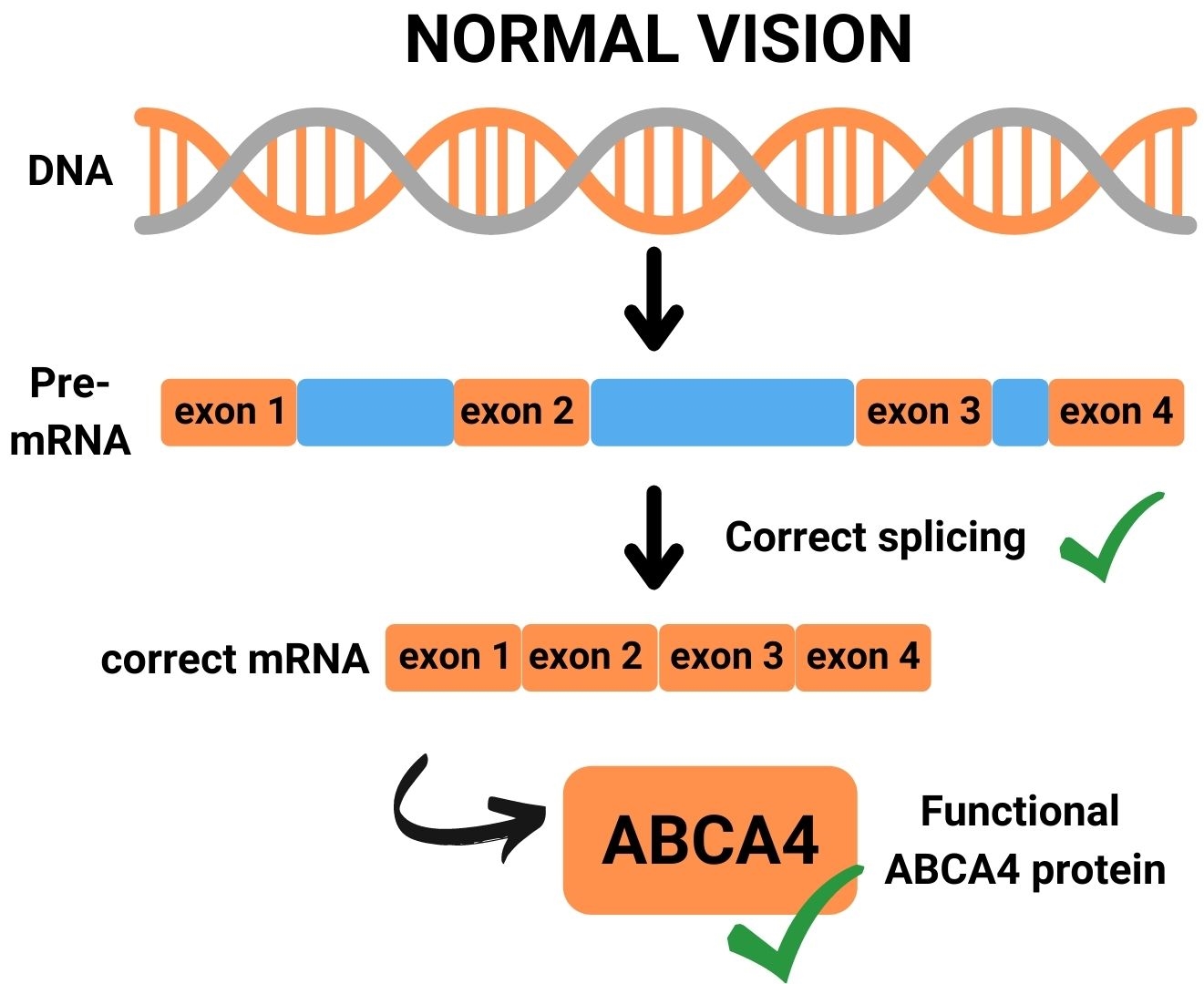
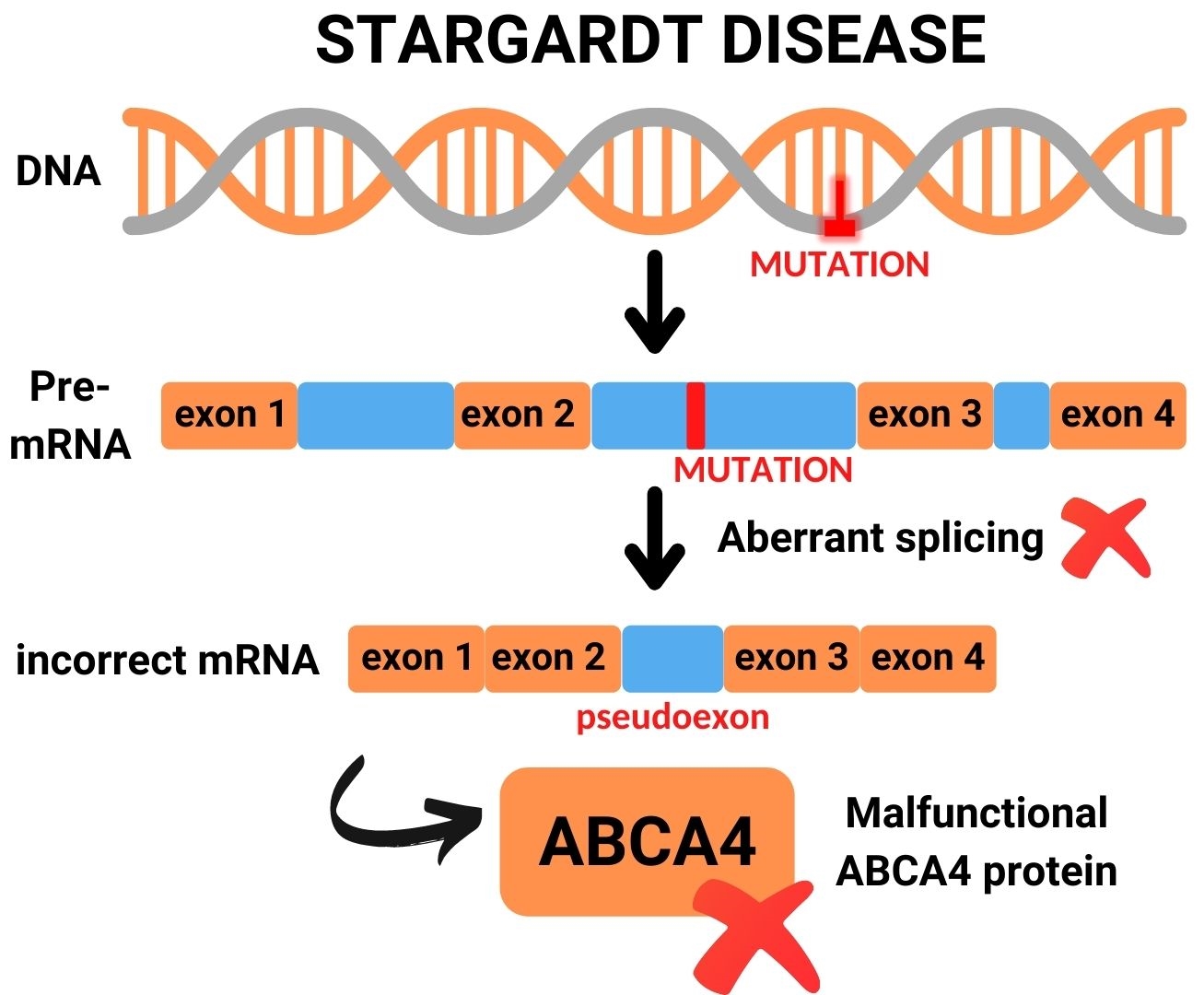
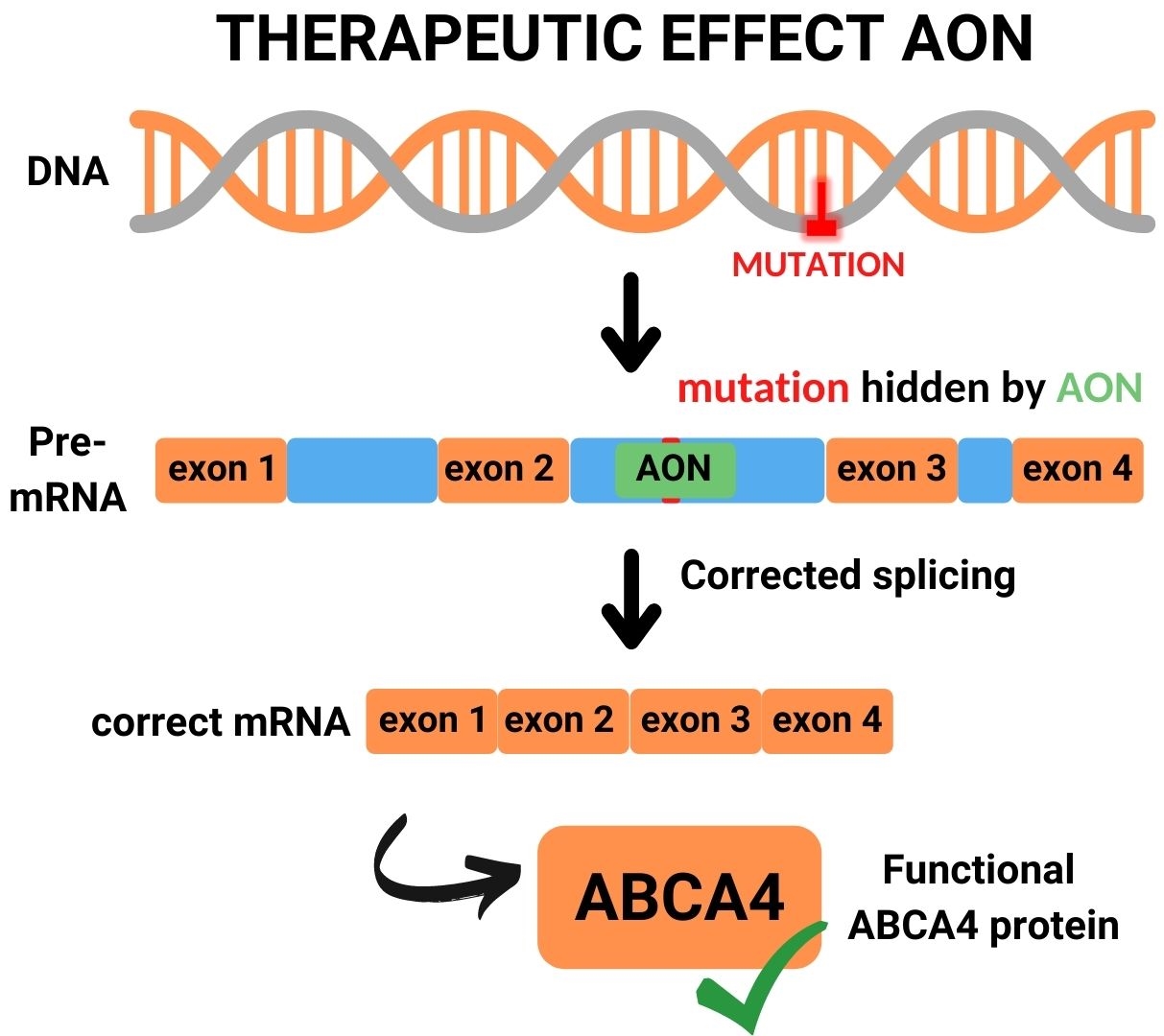
Many deep-intronic mutations in ABCA4 lead to the activation of cryptic splice acceptor sites, cryptic splice donor sites or exonic splice enhancers, all resulting in the insertion of a pseudoexon that in the majority of cases leads to premature termination of protein synthesis. AONs can be designed to block pseudoexon recognition, and thereby restore proper RNA and protein synthesis.
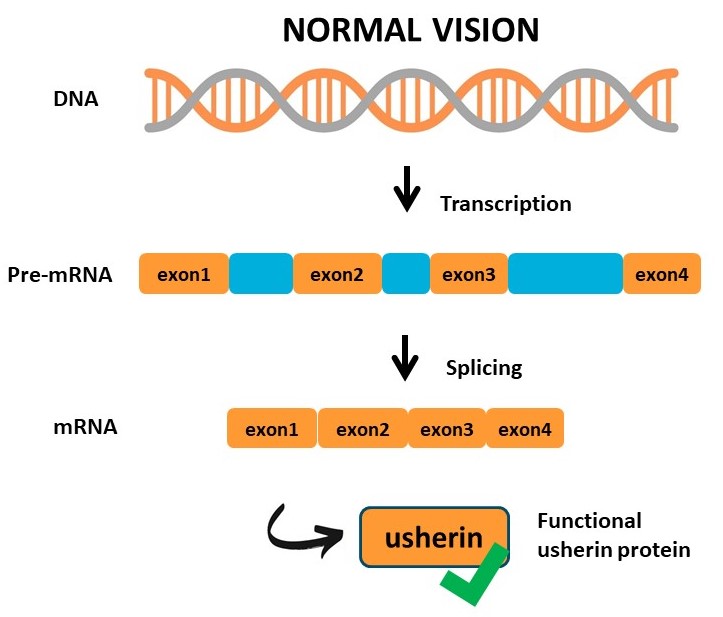
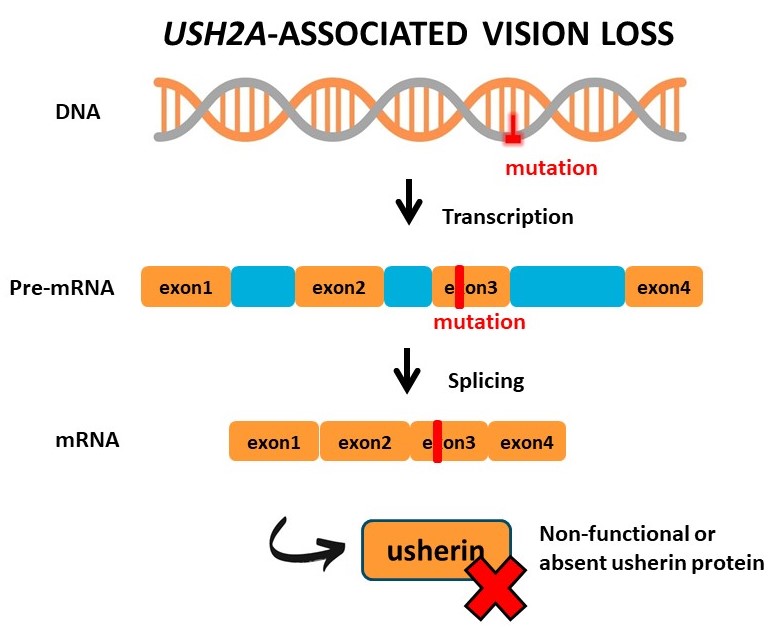
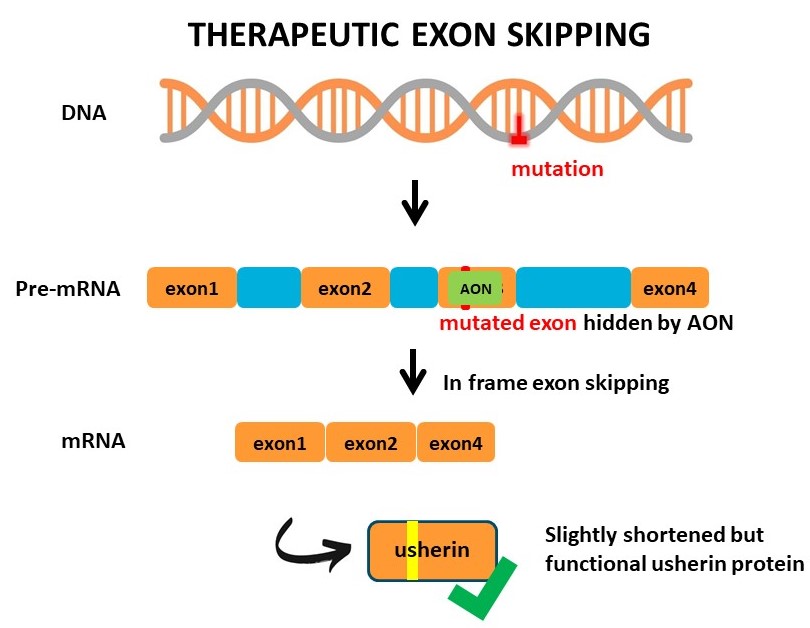
Loss-of-function mutations in the coding region of USH2A result in Usher syndrome type 2A or non-syndromic retinitis pigmentosa. These mutations result in the premature termination of protein translation or misfolding of the usherin protein. AONs designed to block the incorporation of these mutated exons during pre-mRNA splicing, result in the translation of a slightly shortened usherin protein with enough residual function to maintain visual function and photoreceptor integrity. In addition to relatively straightforward single-gene IRD’s, AONs can further be applied to modulate gene expression in multifactorial retinal diseases.

The potential of AON therapy for Stargardt disease and Usher syndrome
Targeting splicing defects in ABCA4 with AONs provides an excellent opportunity to specifically rescue aberrant RNA processing, with the means to treat a significant part of the collective patient pool and prevent further visual impairment in thousands of patients with STGD1 worldwide. For the initial development, we often use patient-derived body material (blood or a skin biopsy) combined with cutting-edge technology to generate cellular models that mimic certain disease characteristics. For USH2A-associated retinitis pigmentosa, targeted skipping of mutated exons has high therapeutic potential for patients having mutations in carefully selected exons. During pre-clinical development, we make use of the powerful combination of zebrafish models and patient-derived 3D organoid models to show therapeutic efficacy.